Washington -Fedient authorities authorized a brand-new sort of medicines on Thursday, which intends to get rid of and Opioid Drugs and various other medicines such as Vicotine and hydroxycin.
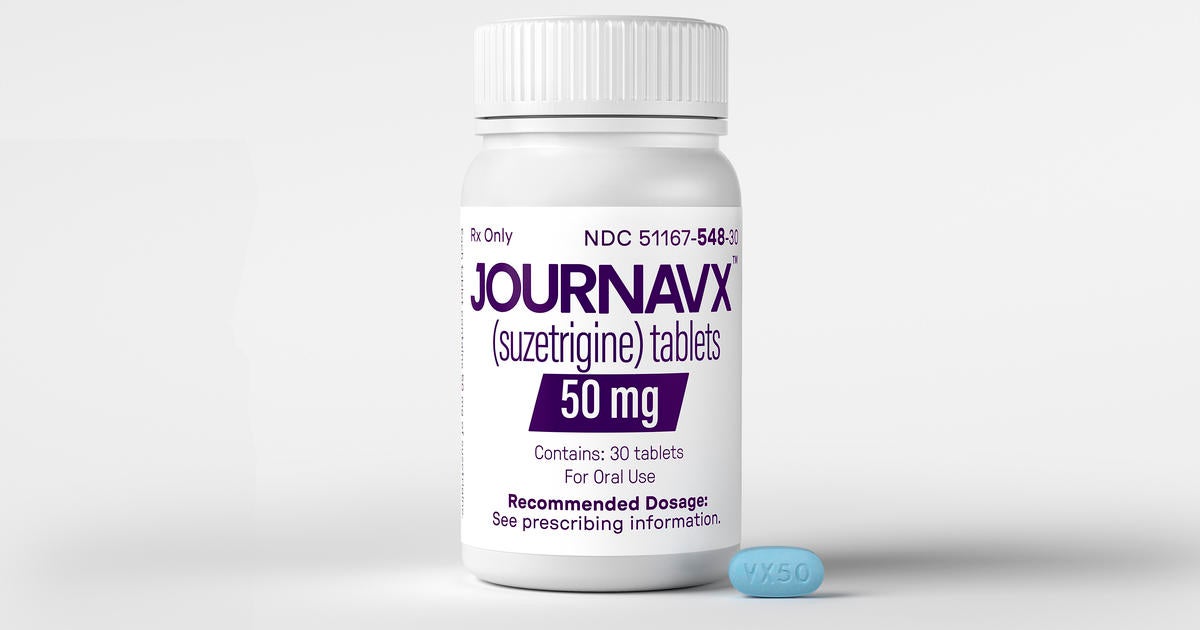
The United States Fda mentioned that it authorized the brief -term discomfort of the Vertex Pharmaceuticals medication Journavx, which was generally executed after surgical procedure or injury.
This is the initial brand-new medicine that has actually been treated with discomfort in greater than twenty years. It supplies choices for opioids and non -prescription medicines, such as advil and acetamine. Nevertheless, the modest performance and lengthy advancement procedure of the medicine highlight the difficulty to discover brand-new approaches to regulate discomfort.
Vertex Pharmaceuticals through AP.
2 research studies from 870 clients with sharp pain were executed after foot and stomach surgical procedure that the medicines of the peptic given extra alleviation than digital medicines, however it did disappoint average opiocene acetaminophenol.
Mayo Schuh, a pharmacologist and discomfort clinical specialist that did not take part in the research, claimed: “This is not a bang dunk for performance.” “However this is a bang dunk since this is a really various method And the engine, so I believe this reveals a great deal of hope.”
Vertex started to examine the medicine in the 1920s. During that time, too much medicines increased, generally by huge -range prescriptions of opioid analgesic medicines, such as usual conditions such as joint inflammation and neck and back pain. The prescription has actually gone down dramatically in the previous 10 years, and the existing opioids are generally due Prohibited fentini Not a medication.
Oed medicines minimize discomfort by binding to the mind receptor that gets nerve signals from various components of the body. These chemical communications can additionally create an addicting impact of opioids.
The function of the medicine’s medicine is various, which stops the healthy protein that causes the discomfort signal, and these healthy proteins are later on sent out to the mind.
Dr. Vertex’s David Altshuler informed the Associated Press in 2015: “When attempting to create medicines with habit forming dangers without opioids, a vital variable is to strive to avoid discomfort prior to the discomfort signal spread.”
The adverse effects of this medicine generally report are queasiness, irregular bowel movements, itching, breakout and frustration.
Dr. Charles Argoff of the Olbani Medical Facility claimed: “New medicines have adverse effects, not just naturally various, however additionally do not include various other essential adverse effects of the danger of misuse of medicines and various other essential adverse effects connected to opioid medicines.” create.
The first principle of concentrating on discomfort signal healthy protein was not conscious discomfort from those entailing unusual hereditary conditions.
Vertex has actually drawn in passion in Wall surface Road since its enthusiastic pharmaceutical pipes include the winning FDA authorization to win a selection of medicines in numerous kinds of persistent discomfort, which generally stands for higher economic chances than sharp pain.
Nevertheless, the supply cost of this Boston pharmaceutical supplier plunged in December. During that time, the frustration of the peak created the study on the back and legs of persistent nerve discomfort. The research discovered that the efficiency of the medicine was not considerably greater than the sugar pill.
Biotechnology expert, Brian Abrahams, claimed in a summary of capitalists: “Our company believe that information shows virtually the most awful instance of this essential network strategy.” He included that the outcome finished completion Vertex’s pipes might deserve greater than billions of kinds of discomfort.
Vertex execs claimed they intended to carry out brand-new message -study on the medicine, to make sure that the concept thinks that various examination layout can create far better outcomes and lead the way for FDA to authorize persistent discomfort.